AEROGEL - The King of Insulation
Aerogel Insulation: Remarkable Insulating Material of the Future

We are in the midst of a new race to explore outer space, and new materials are at the forefront of technological advancement. Consider the needs of a spacesuit. It must protect the astronaut from the extreme temperatures of space, yet be as thin and light as possible to aid in maneuverability.
NASA developed aerogel insulation materials for use in space exploration, but in recent years, aerogels have become commercially available and are finding uses in a variety of other areas.

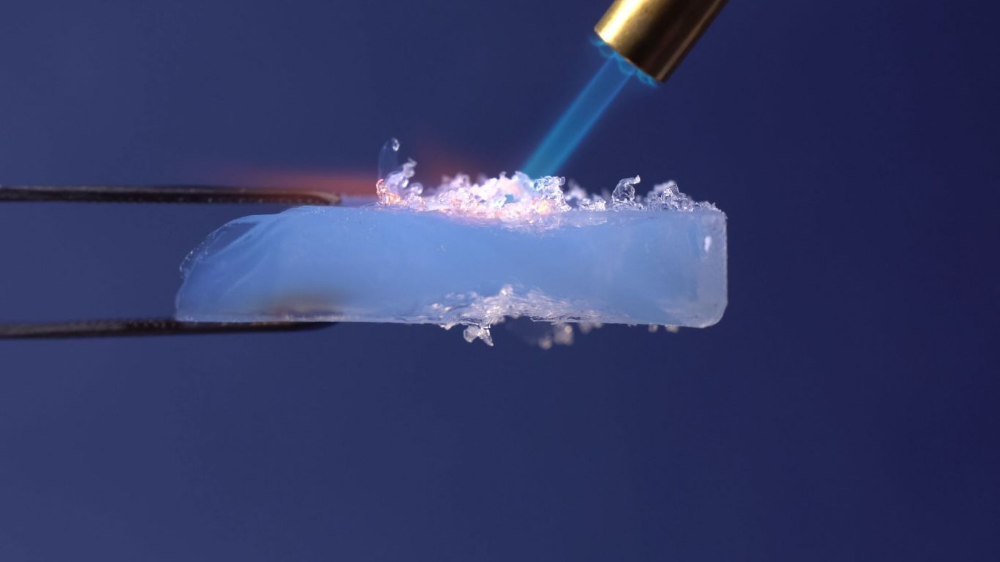
Aerogels are excellent thermal insulators. Here, a piece of aerogel protects a flower from a blowtorch.
Aerogels are advanced materials that, due to their ultra-porous structure, allow engineers to design new thermal insulation for space suits and vehicles and filters, batteries, solar heat collectors, and more.
However, “aerogels” are not a type of material. Rather, they are a special form of solid that can be made from silica, polymers, oxides, carbon, and other materials. Though aerogels are solid, they contain so many tiny voids, or “pores,” that they are mostly composed of air.
In this article, we will answer the following questions:
- What is an aerogel?
- How to make aerogels?
- What are the key properties of aerogels?
- What are the applications of aerogels?
What is an aerogel?
Aerogels are ultra-porous materials, which means that they are full of tiny air-filled holes called pores although they are solid. Those pores are the key to aerogels’ unique properties. While many materials are porous, such as foams and certain ceramics, aerogels are an extreme case.
In aerogels, pores make up most of the material, resulting in an ultra-light solid material. The pores in aerogels are also extremely small, far smaller than human hair, and too tiny to be seen with the naked eye. As a result, aerogels are so light and translucent that they have nicknames like “solid cloud” and “frozen smoke”.
What are the key properties of aerogels?
Aerogels are so porous that up to 95% of their volume is air, which grants them a variety of unusual properties. Amongst these is the fact that they are amid the lightest materials ever made or discovered, which makes them especially useful in aerospace applications where weight savings is vital.
Aerogels’ properties include:
- Extremely low density (see a Matmatch search for low density)
- Very low thermal conductivity (see a Matmatch search for low thermal conductivity)
- High surface area for catalytic or electrochemical reactions
- Translucency (See a Matmatch search for optical transmittance)
The key to these unique properties is the fact that aerogels are not only highly porous, but the pores are also extremely small – too small to see with the human eye. This means that aerogels not only benefit from the low thermal conductivity of the air inside the pores but also the air cannot flow easily, which further enhances their abilities as thermal insulators.
How to make aerogels?
Despite their name, aerogels are not gels, they are highly porous solids that are mostly composed of air. Aerogels start as a liquid, are transformed into a gel, then the liquid is removed. Their unique pore structures are created by preserving the structures created when tiny particles are bonded together in the liquid phase. (See a Matmatch search for small particle size)
The trick is to remove the liquid while preserving the space between the particles. Those spaces become the pores in the aerogel.

The most common type of aerogels are made from silica by the “Sol-Gel” process:
- A “Sol” is made by mixing tiny solid particles with a liquid solvent.
- The Sol is made into a “Gel” by adding a catalyst that bonds the particles to one another, causing the mixture to solidify.
- The liquid solvent is removed by drying, leaving only the solid aerogel behind.
The processing of aerogels is vital to creating their unique microstructure. Without the ultra-small pores leftover from their semi-liquid gel phase, aerogels would not have such low densities or be such excellent thermal insulators.
What are the applications of aerogels?
Aerogels are not a specific material, rather they are a form of material that has been processed to make it extra porous. The most common aerogels are made from silica (silicon dioxide); but there have also been aerogels made from graphene, iron oxide, polymers, and more.
Aerogels also appear in a variety of forms including thick bricks, flexible sheets, and thin coatings. Aerogels can be used in a range of applications, and are commercially available for use as insulation. However, researchers have been developing several other technological applications for these remarkable materials.
Aerogel insulation
The low thermal conductivity and low density of aerogels make them an excellent insulation material. As a bonus, aerogels are so light that they add barely any weight to the structure, which makes them perfect for space travel because every kilogram costs money to lift into space. Aerogels are also excellent insulators that can be used in thin layers where flexibility is needed, such as in space suits.

Some aerogels are translucent, which means that they can be used in places where traditional insulations could not, such as windows and solar panels. Whether they are used for skylights in terrestrial buildings or the windows in a future space habitat, aerogels transmit light but block the transfer of heat.
This makes them ideal for making structures easier to heat and cool while also letting in more natural light. Aerogels have also been used as coatings in next-generation solar heat collectors, where the aerogel allows light to pass through but prevents heat from escaping.
Aerogels’ ability to stop the flow of heat also makes them useful as a form of camouflage, and aerogel coatings have been tested as a way to hide from infrared cameras.
Adsorbers & filters
The tiny pores inside aerogels grant them an especially high specific surface area, meaning that a large amount of the solid material is in contact with its surroundings. When aerogels are made from materials that attract and stick to certain molecules or particles, they can be used as filters and adsorbers that trap substances inside the pores.

Silica gel is an excellent desiccant and chemically safe to use with foods.
A familiar relative of aerogel adsorbers is silica gel, which is commonly used as a desiccant to remove water from the air. Most people are familiar with silica gel packets used to keep food and other items dry, in air conditioning systems, and other applications. After silica gel has been saturated with water, it can be “recharged” by heating in an oven, which vaporizes the water from the surfaces of its pores leaving it dry and ready to use again.
The amount of water that can be absorbed increases with the specific surface area. Since aerogels have an even higher specific surface area than traditional silica gels, aerogels offer even greater improvements in their ability to trap moisture.
Advanced technology applications
The high specific surface area of aerogels means that they have an especially large amount of material in contact with their environment. This makes aerogels useful for a large number of chemical and electrochemical processes that can be improved by maximizing the contact area between solutions and solid substrates.
These include serving as catalysts or catalyst substrates for a variety of industrial chemical processes as well as electrodes in next-generation supercapacitors.

NASA used an aerogel to trap space dust particles aboard the Stardust spacecraft. The particles vaporize on impact with solids and pass through gases, but can be trapped in aerogels. NASA also used aerogel for thermal insulation of the Mars Rover.
Because aerogels are a type of structure that can be made out of a wide variety of materials, researchers are constantly developing new aerogels and new ways to use them. As additional new materials are turned into aerogels, they will enable new technological developments like new supercapacitors, antimicrobial coatings, oil spill absorbing pads, bone implants, and more.
Due to their unique combination of low thermal conductivity, low density, high surface area, and translucence, aerogels are evolving into a diverse array of cutting-edge material technologies.
BY: Wade Lanning -Ph.D. in Materials Science and Engineering